Our group studies metabolic pathways in various tissues and organs of humans and animals. The main focus is the role of energy metabolism in insulin resistance, diabetes mellitus and non-alcoholic-fatty liver diseases (NAFLD) to contribute to an integrative view of the biology of type 2 diabetes (Roden & Shulman. Nature 576:51-60,2019 – 150 Anniversary Collection).
We aim to explore:
- the impact of nutrients on insulin signaling and metabolic fluxes
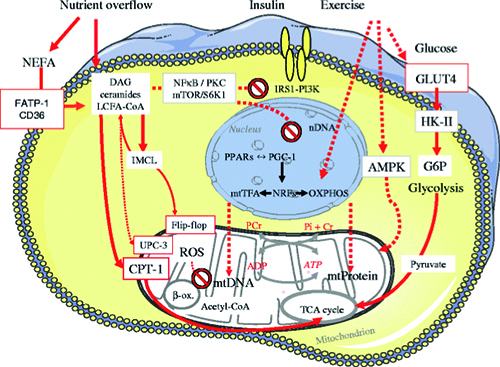
- the regulation of features of the function of mitochondria, the cells’ powerplants, in skeletal muscle, liver and other tissues
- the mechanisms underlying the development and progression of non-alcoholic-fatty liver diseases (NAFLD) in the context of the metabolic diseases
- the of effect lifestyle intervention and drug treatment for the prevention or management of metabolic diseases
- the opportunities for innovative methods for non-invasive studies of metabolism
We have examined the regulation of tissue-specific insulin sensitivity, glucose transport/phosphorylation, glycogen turnover, gluconeogenesis, triglyceride storage, cellular lipid intermediates and oxidative phosphorylation in persons with normal glucose tolerance and in those with various metabolic abnormalities. In this context, we described e. g. the cellular mechanisms of lipid- and amino acid-induced insulin resistance, the role of glycemic control for hepatic glycogen synthesis, the reduction of muscle and liver ATP synthesis in diabetes mellitus and adaptation of hepatic mitochondrial oxidative capacity in obesity and NAFLD.
In close cooperation with the research groups focusing on magnetic resonance (MR) techniques, first in Vienna and now in Düsseldorf, we have developed and are employing novel methods of multinuclei MR spectroscopy combined with stable isotope tracers for real-time monitoring of metabolic flux rates in human liver, muscle and brain. Consequently, we also develop and test individual biomarkers or biomarker panels to improve detection of metabolic abnormalities.
Our aims are:
the identification and characterization of environmental and (epi)genetic triggers of abnormal substrate fluxes and their contribution to human metabolic diseases. The results aim at the better understanding of the cellular and molecular mechanism and the evaluation of novel concepts for preventing and treating diabetes and its comorbidities.